Identification of Lichen Secondary Metabolites using Thin-Layer Chromatography (TLC)
Frank Bungartz1,2,3, Alba Yánez-Ayabaca3,4,5 y María de los Ángeles Herrera-Campos6
1Biodiversity Integration Knowledge Center, Arizona State University, PO Box 874108, Arizona State University, Tempe, AZ 85287-4108, USA
2Charles Darwin Foundation for the Galapagos Islands, Puerto Ayora, Ecuador
3Instituto Nacional de Biodiversidad (INABIO), Quito, Ecuador
frank.bungartz@asu.edu
4Universidad Central del Ecuador, Ciudadela Universitaria, Jerónimo Leyton s/n, Quito, Ecuador
5Herbario Nacional del Ecuador, Quito, Ecuador
albayanez8@gmail.com
6Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México. Apartado Postal 70-367, 04510 México, Cd. de México, México
mahc@ib.unam.mx
_______________________________________________________________________________________
Table of Contents
- Introduction
- Recipes
- Procedure
- selecting specimens
- record sheet
- preparing the plates
- extracting lichen substances
- spotting the plates
- preparing the solvents
- running the plates
- check for fatty acids
- 1st observation & photo documentation
- charring with sulphuric acid
- 2nd observation & photo documentation
- identification of the spots on the plates
- literature and resources
- other literature
- supplies
_______________________________________________________________________________________
Introduction
In thin layer chromatography lichen substances are first extracted from a small sample of the lichen specimen. This extract is then spotted on a glass or aluminum plate coated with amorphous silica gel. Different solvent systems are applied to “run” the plate in a sealed glass tank. The plate is placed in a tank which contains a small amount of solvent. Capillary action draws the solvent through the amorphous silica coating. The lichen substances are carried along according to their affinity to the various solvents. When the solvent front on the plate has reached a certain height plates are removed from the tank and left to dry. The solvent evaporates and some of the lichen substances may already be visible as faint spots. Sulphuric acid (10%) is then applied to the plate. The plate is left to dry again and afterwards placed in a laboratory oven. At 110°C characteristic color reactions of the lichen substances with the sulphuric acid will develop. Many spots which might previously not have been visible will now show up.
There are several diagnostic features which will help to reveal the identity of the spots, i.e., the lichen substances. First of all the distance traveled in a particular solvent is characteristic for each substance. The distance traveled by a substance can be expressed as the Rf-value (retention value). Absolute values may vary considerably according to slight variation in experimental setup. One common method to overcome this variation is to divide the TLC plates into classes of Rf-values. Control specimens which contain substances with well known Rf-values like norstictic acid and atranorin are thus used to “calibrate” the plates. Another way to overcome experimental variation is the calculation of relative rather than absolute Rf-values:
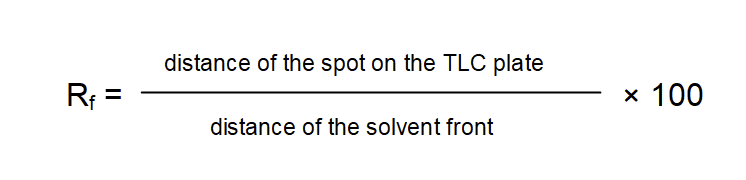
Rf-values are characteristic not only for the specific lichen substances but they also differ according to the solvent used. Atranorin for example has a relative Rf-value of 75 in solvent A but a value of 78 in solvent B’ and 79 in solvent C. Norstictic acid has a relative Rf-value of 40 in solvent A but a value of 32 in solvent B’ and 30 in solvent C. A standardized procedure to distinguish lichen substances therefore uses not only one but preferably two or even three or more different solvents.
Apart from Rf-values, color is an important diagnostic character. The color of the substances usually is considerably different under visible light and UV-light. Many lichen substances have a very characteristic fluorescence under UV-light.
A few substances will already show up on a TLC plate before treatment with sulfuric acid (“charring”), in fact almost all substances are visible as dark spots under short wavelength UV light. Under long wavelength UV-light some of these substances may show a characteristic fluorescence which may change drastically after treatment with sulfuric acid.
_______________________________________________________________________________________
Recipes for various solvents
ATTENTION: All these organic solvents are hazardous! They are highly flammable, easily evaporate and can easily be breathed in. Many are considered cancerogenous. ALWAYS use them ONLY under the fume hood !!!
Routine TLC is typically carried out using solvent C. For reliable results it is recommended, however, to use at least three different solvent systems, typically A, B’, and C.
Please note: The numbers in parentheses below denote the relative proportions. Thus, for example Solvent C can be mixed as 170 ml of toluene with 30 ml of acetic acid; or, if a only smaller quantity is needed as 85 ml of toluene with 15 ml of acetic acid, and, if a larger quantity is needed 340 ml of toluene with 60 ml acetic acid. The proportion ‘170:30’ of course remains the same.
Standard Solvents
A toluene / dioxane / acetic acid (180 : 45 : 5)
Dioxane in solvent A is hygroscopic and absorbs water over time. The solvent thus rapidly deteriorates and accurate Rf-values can only be recorded with relatively fresh solvent. The original formula for this solvent was proposed by Culberson & Ammann (1979).
B hexane / diethyl ether / formic acid (130 : 80 : 20)
This solvent has largely been replaced by B’. B will deteriorate in less than 6 hours whereas B’ can be used up to four or five days.
B’ hexane / methyl tert-butlyl ether / formic acid (140 : 72 : 18)
[B‘ cyclohexane / methyl tert-butyl ether / formic acid (130 : 100 : 20)]
The original formula of B proposed by Culberson (1972) used diethyl ether, a rather hazardous substance and diethyl ether is in B’ therefore now typically replaced by methyl tert-butlyl ether. For high performance TLC [= HPTLC, Arup et al. (1993) suggested to use cyclohexane instead of hexane, again largely for safety reasons].
C toluene / acetic acid (170 : 30)
For most lichen substances C provides the best discrimination. It is also very stable (several weeks) and therefore is the most common solvent routinely used.
Special Solvents
Solvents D, E, F and G are mostly used only to discriminate substances which will otherwise not be resolved very well:
D butanol/ acetone/ water (150 : 30 : 60)
Culberson & Ammann (1979) initially referred to this as solvent system ‘E’. However, the solvent is generally now called D, the letter E typically instead used to denote a solvent introduced later by Elix & Crook (1992). Solvent D is best suited for the separation of secondary metabolites with unusually low Rf-values, e.g., β-orcinol depsidones. Generally this solvent is rarely used and neither the Catalog of Standardized Chromatographic Data (Elix 2014) nor Wintabolites includes Rf-values in D. If necessary the original Culberson & Ammann (1979) must be consulted.
E cyclohexane / ethyl acetate (75 : 25)
Solvent E discriminates between non-polar derivates of lichen compounds and substances which have very high Rf-values in A, B, B’ and C. The solvent helps to discriminate for example atranorin, chloroatranorin, pannarin, physciosporin and several xanthones. It needs to be prepared fresh daily.
F ethyl acetate / cyclohexane (1 : 1)
Solvent F is best suited to separate xanthones, it also can be used to distinguish atranorin from chloroatranorin (Elix & Crook 1992).
G toluene / ethyl acetate / formic acid (139 : 83 : 8)
Solvent G is very stable and discriminates well between substances with very low Rf-values in A, B, B’ and C.
For additional, even more specialized solvent systems, consult Orange et al. (2010).
_______________________________________________________________________________________
Procedure
The whole procedure can be summarized as follows:
(1) Selection of Specimens – (2) Preparation of Record Sheet – (3) Preparation of the plates – (4) Extracting Lichen Substances – (5) Spotting Extracts on the Plates – (6) Preparation of the solvents – (7) Running the Plates in the TLC chamber – (8) Check for Fatty Acids – (9) 1st Photo Documentation – (10) Preparation of 10% H2SO4 & Treatment with 10% Sulphuric Acid & “Charring” – (11) 2nd Photo Documentation – (12) Analysis of the Plates in MYTABOLITES.
If you do not have enough time you can interrupt the process at two stages, after preparing the plates (4) or after spotting the extracts onto the plates (6). You should always mix fresh solvent (6) on the day that you actually run the plates, not the day before! Some solvents rapidly deteriorate. After running the plates (7), you can not interrupt the following steps. To avoid deterioration of your colors and changing how the metabolites react with sulphuric acid it is necessary to take the photos of the plates as soon as possible (9) and then immediately apply the sulfuric acid, followed by charring at 110°C in a laboratory oven (10), followed again by taking photos of the plates after charring (11). Once you have photo documentation of the plates before and after charring, you can work on analyzing your results in the following days. The plates deteriorate, but if you have the photos this is not problematic. Checking the plates for fatty acids (8) is typically necessary only in a few groups, which are characterized by different fatty acids.
Here these steps in more detail …
_______________________________________________________________________________________
(1) Selection of Specimens:
Genera that frequently need thin-layer chromatography for accurate identification are those with a complex chemistry, but fairly similar, very plastic, highly variable or indistinct morphology. For example:
Many species of Cladonia with cup-shaped podetia can be confusingly similar, but their chemistry is typically very distinct. In the Sonoran Region the genus Xanthoparmelia is very common and represented by an enormous diversity; it much helps knowing the secondary chemistry of the specimens to narrow down the correct identification. Finally, many leprose lichens display very little morphological variation. Species of Lepraria differ slightly by their color, the size of their granules, how compact their granules are, but they are typically well separated by their secondary chemistry
_______________________________________________________________________________________
(2) Preparation of Record Sheet:
Fill out the TLC record sheet and clearly mark your specimen packets:
- Write plate #, solvent system and date on top of the form.
- Write specimen# and preliminary identification (if any) into the rows indicating each specimen.
- Write the tube numbers, date and solvent system you are using on the specimen packets from which you are going to take a sample.
Below an example of the Record Sheet; you can also download a template here.
At the ASU Lichen Herbarium we use a mix of different species as our control. This mix of different species contains a range of secondary metabolites ranging across the spectrum of Rf-values in most solvents (e.g., in solvent C the folowing substances ordered by their Rf-values: 2 = constictic acid, 3 = connorstictic acid, 4 = salazinic acid, 10 = cryptostictic acid, 13 = 3-hydroxyphysodic acid, 18 = physodic acid, 18 = stictic acid, 19 = galbinic acid, 30 = norstictic acid, 70 = usnic acid, 79 = atranorin).
This later facilitates calibrating the TLC plates (see the cuning procedure in MYTABOLITES).
Below an example of some specimens that contain a range of secondary metabolites together covering a large spectrum of Rf-values (for the control mix you need only minute pieces that you can grind up into a fine powder in a mortar):
Hypogymnia physodes – 2′-O-Methylphysodic acid, Atranorin, Chloroatranorin, Physodalic Acid, Physodic Acid, Protocetraric acid
Hypotrachyna microblasta – Usnic acid, ±Atranorin, Norstictic acid, Galbinic acid, Salazinic acid, Consalazinic Acid, ±Gyrophoric Acid
Hypotrachyna microblasta – Usnic acid, ±Atranorin, Norstictic acid, Galbinic acid, Salazinic acid, Consalazinic Acid, ±Gyrophoric Acid
Parmelia sulcata – Atranorin, Consalazinic Acid, Pseudoplacodiolic Acid, Salazinic Acid
Parmotrema crinitum – Atranorin, Chloroatranorin, Constictic Acid, Cryptostictic Acid, Menegazziaic Acid, Stictic Acid, 9a-Hydroxymenegazziaic Acid, 3-O-Methylconsalazinic Acid
Physcia adscendens – Atranorin
etc.

_______________________________________________________________________________________
(3) Preparation of the plates:
Use only soft pencil, else the silica will be damaged and once badly grooved the plates are useless !!!
We will be using the smaller 10×10 cm HTLC plates according to Arup et al. (1993) with solvent C (if there remains sufficient time possibly also solvent B’ and A).
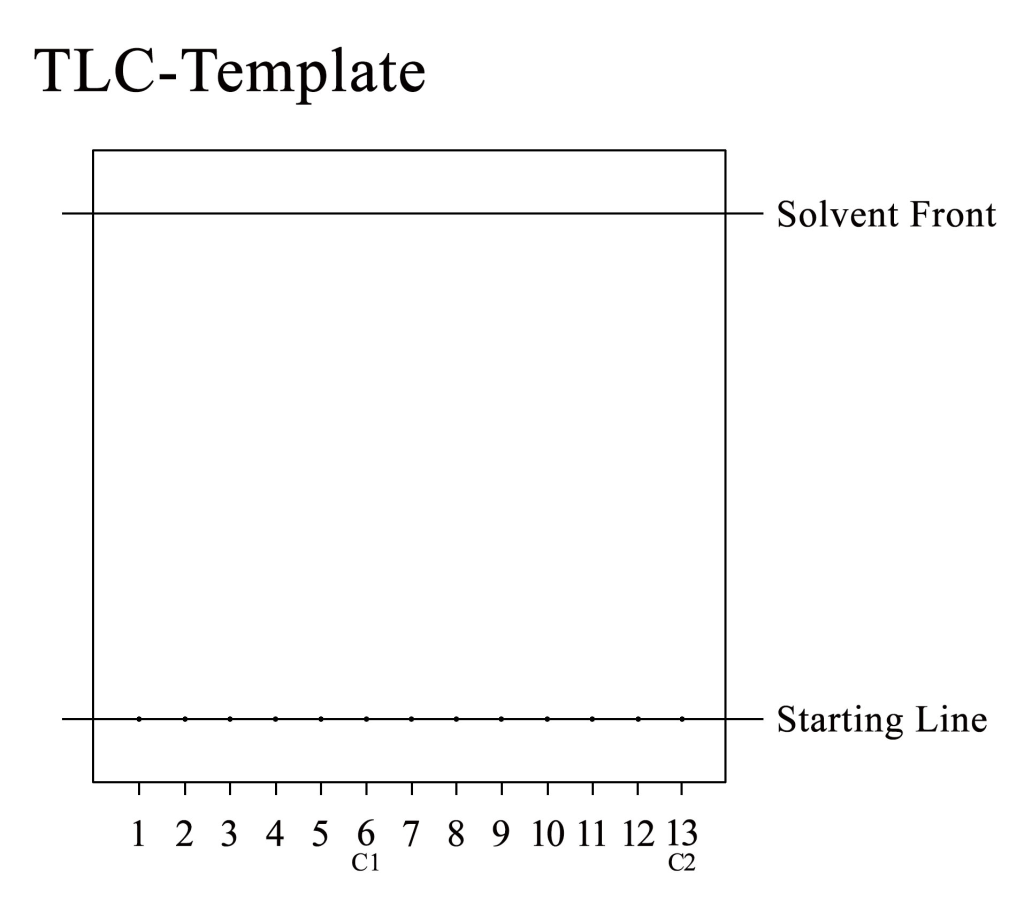
- Draw a line 1 cm above the bottom of the plate. This lower line represents the “starting line”. You will spot the extracts of lichen substrates onto this line.
- Mark the lower line with 13 spots, each 7 mm apart; spot number 6 at the center of the plate will be your control #1 (C1) and spot number 13 at the right margin your control #2 (C2).
- Draw a second line 1 cm below the top. This upper line marks the “maximum” solvent front, i.e. how far you will let the solvent run up the plate. When the solvent has reached this line, take the plate out of the chamber immediately. NEVER let the solvent front reach the top of the plate !!!
- Mark the plate above the solvent front line with a plate number, date, your name and the solvent system you are going to use (we will be using solvent C).
_______________________________________________________________________________________
(4) Extracting Lichen Substances:
- Label thirteen Eppendorf tubes (1-13). Mark #6 and #13 as C1 & C2 respectively to indicate that they are your control substances.
- Place the Eppendorf tubes into a rack.
- Carefully remove a small sample of your lichen (with forceps or scratch a sample of the rock substrate) and place it into the labeled tube. If you have several thalli growing in close proximity be extremely cautions not to include even tiny thallus fragments from adjacent thalli!!! You only need a few square mm, perhaps 3-5 areoles of a crustose lichen, a few lobules of a foliose one or some small pieces of the branches of a fruticose specimen.
- Place a tiny amount (one or two drops) of acetone in each sample tube. Your specimen should barely be covered with acetone.
_______________________________________________________________________________________
(5) Spotting Extracts on the Plates:
Leave the Eppendorf tubes open so excess acetone can evaporate. After two to three minutes you can use a capillary tube to spot your acetone extract onto the TLC plate:
- Just immerse the capillary tube into the sample tube. Acetone extract will be drawn into the tube by capillary action.
- Place your finger on top of the tube, so the acetone does not run out of the tube, when you place it onto the corresponding spot on the TLC plate.
- Carefully spot the capillary tube onto the mark on your TLC plate. Don’t let too much extract be drawn onto the plate. The smaller your spots on the plate are the less likely they will float into each other when they run up the plate.
- Repeat the procedure until you can clearly distinguish a spot of lichen substance on the plate.
_______________________________________________________________________________________
(6) Preparation of the solvents:
ATTENTION: All these organic solvents are hazardous! They are highly flammable, easily evaporate and can easily be breathed in. Many are considered carcinogenic. ALWAYS use them ONLY under the fume hood !!!
Mix the solvent(s) that you need according to the formulas above. As a standard for routine TLC we will use solvent C, i.e., toluene with acetic acid in a relation of 170 to 30 (HTLC uses relatively small amounts, for example 170 ml + 30 ml).
Recipes for the different solvents can be found here.
Solvent C is often used routinely, because it generally provides good discrimination of most lichen secondary metabolites. However, to accurately distinguish different metabolites, running at least three plates in different solvents (A, B’, C) is often necessary.
_______________________________________________________________________________________
(7) Running the plates:
At the ASU Lichen Herbarium we use horizontal developing chamber from CAMAG for 10×10 cm HTLC plates:
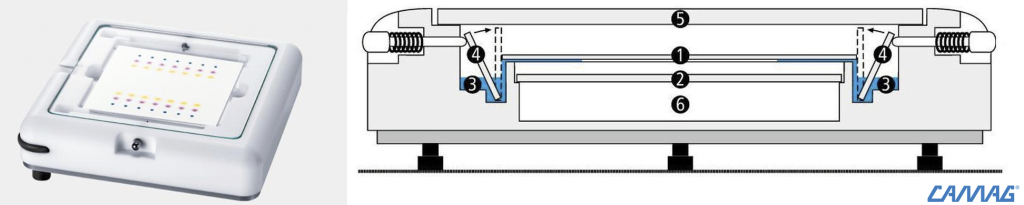
| CAMAG HTLC chamber | Schematics of the HTLC chamber: (1) HTLC-plate, (2) glass plate, (3) solvent, (4) glass strip to start the process (by flipping it towards the plate, the solvent touches the plate and begins running through the silica gel), (5) cover glass plate, (6) lower chamber |
- Place the HPLC chamber into the fume hood and balance the chamber with the level so it is perfectly plane (this is important so that the solvent runs evenly through silica coating of the plates).
- Fill the bottom groove of the flat solvent tank; just add sufficient solvent that it barely fills the groove, make sure the glass starter are flipped backwards.
- Carefully insert the plate.
- Cover the TLC chamber.
- Push in the pin to flip over the glass starter strip so the solvent now touches the TLC plates and begins running through the plate.
- Push in the pin to flip over the glass starter strip so the solvent now touches the TLC plates and begins running through the plate.
- Let the solvent run through the plate; it will run between 15 to 30 min until the solvent front reaches the top line (CHECK after 15 minutes !!!)
- Take off the lid and take out the plate. NEVER let the front run over the top line !
_______________________________________________________________________________________
(8) Check for Fatty Acids (useful for some species):
Important: Make sure that all residue from the solvents has evaporated under the fume hood. Never handle the plates when they still smell from solvent. Many are considered carcinogenic.
Strictly speaking, fatty acids are not secondary metabolites, but for some species groups (e.g., Peltigeraceae) they have been found to be taxonomically relevant. Fatty acids are carried along by the solvents across the plate, but they do not show up in visible or UV-light.
To make fatty acids visible spray the plate with water. Fatty acids are not soluble in water and therefore will show up as pale spots, where the water doesn’t penetrate the silica gel on the plates. To mark where these fatty acid spots show up, circle and cross spots with a soft pencil (be extra careful not to scratch the silica gel surface; when wet you can easily damage the silica).
Let the plate dry before the next step.
_______________________________________________________________________________________
(9) First Observation & Photo Documentation of the Plates:
Once the plates are dry and any solvent residue has evaporated, observe and photograph them under UV light. Different wavelength give different results:
λ254 nm: Take a photograph of the plate under short wave UV-light (λ254 nm); the plates have a bright green fluorescent indicator and any spots not visible in normal light, can be seen as dark spots against the bright green background.
λ365 nm: Take a photograph of the plate under long wave UV-light (λ365 nm); some but not all of the secondary metabolites will already display their characteristic fluorescence.
Photodocumentation is an excellent way to preserve the colors of the different spots. Within only few days any colors will fade or darken. It is much easier also to compare the colors under different light, before and after charring. This takes a lot of guesswork out of interpretation of your results (before digital photography was widely available, researchers typically used pencil markings and letter codes to mark the colors on the plates).
_______________________________________________________________________________________
(10) Charring the plate with 10% Sulfuric Acid:
ATTENTION: diluting acid is an exothermic reaction, therefore always add the acid to the water, not the other way around !!!
In a wide-neck jar, dilute the concentrated sulfuric acid down to 10% (e.g., 98% sulfuric acid of a density 1.84 and distilled water in the proportion 1: 9).
10% sulphuric acid will keep for many months and does not need to be prepared fresh every time. However, it will aggressively deteriorate the brushes that you are using. These need to be rinsed thoroughly with water immediately after applying the acid !!! Also, since you are using cheap and easily replaceable paintbrushes, some of the acid reacting with the bristles will eventually spoil your acid solution and it is then necessary to mix fresh acid again.
- Brush the plates with 10% Sulfuric Acid and let them dry.
- Place the plates into the oven at 110°C for approximately 5-8 min (if you have a lower temperature oven, try increasing the time)
- Check color development !!! The color should appear bright and well developed but not burned.
- Take the plate out and let it cool down.
_______________________________________________________________________________________
(11) Second Observation & Photo Documentation of the Plates:
Take a photograph of the plate in visible light; after treatment 10% Sulfuric Acid the characteristic colors should be well pronounced, they again fade or become dull with time.
Take a photograph of the plate under long wave UV-light (λ365 nm); again the color show their most characteristic fluorescence as soon as the plates have cooled down and they fade with time.
Now you are ready calculate your Rf-values and feed your data into MYTABOLITES !!!
An example of the photo documentation of a finished plate below:

_______________________________________________________________________________________
(12) Identification of Spots on the Plate:
To identify the different spots on the TLC plates, you are now ready to use Mytabolites to calculate the Rf-values and enter the color of the spots after and before charring.
_______________________________________________________________________________________
Literature and Resources
The procedure outlined above mostly follows the procedures in Orange et al. (2010), using the program Mytabolites for the analysis of the plates; that program includes all data also available in the Catalogue by Elix (2022):
Orange, A, James, PW & White, FJ (2010) Microchemical methods for the identification of lichens, second edition with additions and corrections. British Lichen Society, London. Second edition with additions and corrections. Published by the British Lichen Society, available for download here.
Elix, JA (2022) Catalogue of standardized chromatographic data and biosynthetic relationships for lichen substances. Sixth Edition. Published by the author, Canberra, available for download here.
Lafferty, D., Bungartz, F., Elix, J.A. & Schumm, F. (2024) Mytabolites – a program developed at Arizona State University for the interpretation thin-layer chromatography plates, for the analysis of secondary metabolites from lichens, based on an original concept published by E. Mietzsch, H.T. Lumbsch & J.A. Elix. Help & Resources for the Consortium of Lichen Herbaria, available at https://help.lichenportal.org/index.php/en/resources/metabolites/
Detailed instructions about MYTABOLITES with the download link are available here.
_______________________________________________________________________________________
Other Literature
Arup U, Ekman S, Lindblom L, Mattsson JE (1993) High performance thin layer chromatography (HPTLC), an improved technique for screening lichen substances. Lichenologist 25(1): 61-71.
Culberson CF (1969) Chemical and botanical guide to lichen products. Chapel Hill (University of North Carolina Press).
Culberson CF (1972) Improved conditions and new data for the identification of lichen products by a standardized thin-layer chromatographic method. Journal of Chromatography 72: 113-125.
Culberson CF (1976) Supplement to chemical and botanical guide to lichen products. The Bryologist 73: 177-377.
Culberson CF, Culberson WL, Johnson A (1981) A standardized TLC analysis of ß-orcinol depsidones. The Bryologist 84: 16-29.
Culberson CF, Johnson A (1982) Substitution of methyl tert-butyl ether for diethyl ether in the standardized thin-layer chromatic method for lichen products. Journal of Chromatography 238: 483-487.
Culberson CF, Kristinsson HD (1970) A standardized method for the identification of lichen products. Journal of Chromatography 46: 85-93.
Culberson, CF (1969) Chemical and botanical guide to lichen products. Chapel Hill. University of North Carolina Press.
Culberson, CF, Ammann, K (1979) Standardmethode zur Dünnschichtchromatographie von Flechtensubstanzen. Herzogia 5: 1-24.
Culberson, CF, Culberson, WL, Johnson A (1977) Second supplement to chemical and botanical guide to lichen products. Missouri Botanical Garden. St. Louis.
Elix, JA (2022) Catalogue of standardized chromatographic data and biosynthetic relationships for lichen substances. Sixth Edition. Published by the author, Canberra, available for download here.
Huneck S, Yoshimura I (1996) Identification of Lichen Substances. Springer, Heidelberg.
Mietzsch E, Lumbsch, HT & Elix, JE (1994): WINTABOLITES (Mactabolites for Windows). Users manual and computer program, 2nd ed. (Universität Essen) 54p (The reference to the original version of WINTABOLITES, a computer program to interpret TLC-plates).
Nylander W (1867) Hypochlorite of lime and hydrate of potash, two new criteria in the study of lichens. Linnean Society’s Journal of Botany 9: 357-365.
Orange A, James PW, White FJ (2001) Microchemical methods for the identification of lichens. British Lichen Society. First edition.
Orange A, James PW, White FJ (2010) Microchemical methods for the identification of lichens. British Lichen Society. Second edition with additions and corrections.
White FJ, James PW (1985) New Guide to microchemical techniques for the identification of lichen substances. Bulletin British Lichen Society 57: 41.
_______________________________________________________________________________________
Supplies
Please Note:
Do not do this in your kitchen !!! The solvent fumes are toxic, causing cancer and you must use a fumehood for adequate ventilation. Make sure the plates have completely dried and all solvent has evaporated before inspecting them.
The list below is meant as orientation. We do not endorse particular vendors. You can buy all supplies from vendors different from the ones listed here.
Traditionally, thin-layer chromatography uses large upright glass tanks designed for 20 x 20 cm silica coated plates. These tanks are much cheaper than the CAMAG horizontal chamber recommended below. The the 20 x 20 cm plates are also cheaper. However, the amount of chemicals needed is significant. For the CAMAG chamber you will use ca. 3 mL of solvent for each run. This is not just a lot less hazardous, but you can save a lot of money buying solvents less frequently (after several hundred plates, we still did not have to by new solvents at ASU). Thus, the investment in the horizontal chamber and smaller, fine-grained plates will easily be compensated by the much less solvent used.
For chemicals needed, make sure to buy the purest grades available to avoid contaminations that can be confusing when interpreting the plates. Generally speaking, any band that always occurs across all samples across different plates is most likely not a lichen secondary metabolite, but caused by impurities of the solvent.
These solvents as well as sulfuric acid are hazardous and in most countries can be purchased only with a license.
The list below does not include costs! Costs will vary from vendor to vendor, country to country, and of course prices increase with time [as of 2026 a rough estimate to at least get started is around $3,000 minimum (chamber, lamp, small packet of plates, small quantities of solvents, perhaps only use solvent C), more realistically a complete setup needs a budget between $5,000 to $6,000].
- CAMAG horizontal developing chamber for high performance thin-layer chromatography (HPTLC)
for 10 x 10 cm plates (part #022.8530) - Camag UV-lamp with dual wavelength (254/366 nm); best to purchase only the lamp and not the CAMAG UV-cabinet sold separately (you can easily build a viewing chamber from a wooden box that you simply paint black inside and attach a camera to take photos of the plates; the CAMAG chamber is not ideal if you want to attach a camera)
- digital camera to take images of the plates under natural light and UV
- laboratory oven [with capacity to be heated to 100-110°C (210-230°F)]
- HPTLC glass plates, coated with silica gel 60 matrix, 10 x 10 cm, with fluorescent indicator for 254 nm UV-light, e.g.:
HPTLC Silica gel 60 F254 (Merck 1.05629.0001); in the US supplied for example by SigmaAldrich - optional: ziploc bags & silica desiccant [especially in humid climates, when the workflow has to be unexpectedly interrupted, it is recommended to temporarily store plates in sealed plastic bags with a desiccant; silica beads with orange indicator are non-toxic, they turn pale yellow absorbing humidity, then can be “refreshed” and reused by drying in a laboratory oven (90°C or 200°F)]
- Drummond 10 µL “Microcaps” disposable micro-pipettes (use each pipette for only one sample & discard)
- soft pencil for marking the plates (B; don’t use HB, too hard and will scratch the plates)
- record sheet (template here)
- metric ruler (for marking the plates)
- 50 or 100 mL dropper bottle (for acetone)
- safe-lock 0.5 mL Eppendorf-tubes (these smaller tubes are typically used for PCR, but they work much better with the tiny micro-pipetes than regular Eppendorf-tubes)
- Eppendorf microtube rack for 0.5 mL tubes
- HPLC-grade acetone (for extricating secondary metabolites from specimens)
- HPLC-grade solvents (which solvents you need depends on the secondary metabolites targeted, see Recipes for Various Solvents; routinely plates are run in C; ideally plates should be run in at least three different solvents, typically A, B’, and C
- glas bottles (for storing solvents; used different bottles for the different solvents)
- sulfuric acid (most economic is to buy concentrated and diluting it to 10%)
- broad jar with lid (for storing 10% sulfuric acid; to be kept inside the fumehood, the mouth of the jar needs to be wide enough for a paint brush)
- broad, cheap paintbrush (use for brushing the plates with diluted sulfuric acid; for repeated use rinse with water immediately after use; discard before bristles disintegrate or start dissolving)
- spray bottle (for wetting plates to detect fatty acids; do not spray sulfuric acid; although some of the older publications recommend spraying the plates with diluted sulfuric acid, this is VERY hazardous and can easily be avoided by brushing the plates instead)
- for mixing solvents use calibrated glass pipettes with bulbs; some people prefer graduated glass cylinders (ideally separate ones for different reagents; attention: do not use the one for diluting sulfuric acid also for the solvents)
- scalpel for scratching off lichen specimens from their substrate and/or fine forceps to remove individual parts for analysis
- small pieces of scratch paper, folded in half (to collect small samples removed from specimens; the central fold facilitates pouring the material into the Eppendorf-tubes; to avoid cross-contamination use one piece of paper per specimen, then discard)
- small porcelain mortar (for grinding up reference specimens to be used as control)
- Mytabolites (i.e., the software for analyzing the plates)
_______________________________________________________________________________________
